VivaCell platform to analyse and develop products to treat overactive bladder
To study the effects of products on various parameters to be targeted in the treatment of overactive, we designed several objectives/tasks.
Modul Activity 1:
Effects on TRPV1 and CB1
- TRPV1 antagonistic activity
- CB1 agonistic activity
Modul Activity 2:
Effects on inflammatory parameters (human monocytes or urothelial cells)
- PGE2
- CRP
- TNFalpha
- IL-6
- IL-1
Modul Activity 3:
Effects on NK2 receptor induced by NKA/SP
- Antagonistic effects on NK2 receptors
Modul Activity 4:
Muscarinic receptors
- Antagonistic effects on M1 receptors
- Antagonistic effects on M2 receptors
- Antagonistic effects on M3 receptors
- Antagonistic effects on M4 receptors
- Antagonistic effects on M5 receptors
Modul Activity 4:
Purinergic receptors
- Antagonistic effects on P2X3 receptors
Modul Activity 5:
PDE4
- Inhibitory effects on PDE4
Modul Activity 6:
beta-3-adrenergic receptors
- Agonistic effects on beta-3-adrenergic receptors
Overactive bladder (OAB) describes a combination of symptoms that can include a frequent urge to urinate and waking up at night to urinate. Causes can include weak muscles, nerve damage, use of medications, alcohol or caffeine, infection, and being overweight. Disturbances in nerves, smooth muscle, and urothelium can cause this condition. In some respects the division between peripheral and central causes of OAB is artificial, but it remains a useful paradigm for appreciating the interactions between different tissues. Models have been developed to mimic the OAB associated with bladder instability, lower urinary tract obstruction, neuropathic disorders, diabetes, and interstitial cystitis. These models share the common features of increased connectivity and excitability of both detrusor smooth muscle and nerves. Increased excitability and connectivity of nerves involved in micturition rely on growth factors that orchestrate neural plasticity. Neurotransmitters, prostaglandins, and growth factors, such as nerve growth factor, provide mechanisms for bidirectional communication between muscle or urothelium and nerve, leading to OAB with or without urge incontinence.
The transient receptor potential vanilloid subfamily 1 (TRPV1) is an ion channel activated by capsaicin, heat, protons and endogenous ligands such as anandamide. It is largely expressed in the urinary tract of mammals. Structures in which the receptor expression is firmly established include sensory fibers and urothelial cells. As in other systems, pain perception was the first role attributed to TRPV1 in the urinary tract. However, it is now increasingly clear that TRPV1 also regulates the frequency of bladder reflex contractions, either through direct excitation of sensory fibers or through urothelial-sensory fiber cross talk involving the release of neuromediators from the epithelial cells. Desensitization of the receptor by capsaicin and resiniferatoxin has been investigated for therapeutic purposes. For the moment, lower urinary tract dysfunctions in which some benefit was obtained include painful bladder syndrome and overactive bladder of neurogenic and non-neurogenic origin. However, desensitization may become obsolete when non-toxic, potent TRPV1 antagonists become available.
The role of the cannabinoid system in the regulation of bladder function has attracted considerable interest in the last few years. Several studies have reported the presence of cannabinoid receptors CB1 or CB2 mRNA and/or protein in the bladder of humans. The localization of CB1 receptors has been described in the urothelium and in nerve fiber structures of the suburothelium and the detrusor. It has been shown that local activation of CB1 receptors reduces the sensitization of bladder afferent neurons during acute inflammation. In addition, the co-expression of CB1 with TRPV1 in nerve fibers confirms the presence of CB1 in peptidergic nociceptors and can therefore explain the role of cannabinoid agonists in modulating the sensitization of bladder afferents.
Several inflammatory parameters have been shown to be involved in the inflammatory part of OAB. This includes C-reactive protein (CRP) which is produced and secreted by the liver in response to inflammatory processes occurring in the body. The association of elevated serum CRP with various lower urinary tract symptoms (LUTS) suggests a possible role of inflammation.
NGF plays a key role in the survival of sensory neurons during development and is necessary throughout adulthood for maintenance of the normal properties of small-sized afferent neurons with unmyelinated axons (i.e. C-fiber afferents). There is also growing evidence that NGF is a peripheral mediator of several types of inflammatory painful conditions.Prostaglandin E2 (PGE2) synthesized in bladder muscle and mucosa has a complex local action in the bladder. PGE2affects the normal micturition reflex and under pathophysiological conditions (e.g. mucosa injury and inflammatory mediators). Intravesical administration of PGE2 stimulates reflex micturition through activation of capsaicin sensitive afferent nerves and causes bladder overactivity in rats and in humans.
A previous study has suggested the association of inflammation with OAB symptoms by the significant elevation of NGF and PGE2 levels in the urine of OAB patients.
Tachykinins (TKs) are involved in both the physiological regulation of urinary bladder functions and development of overactive bladder syndrome. The aim of the present study was to investigate the signal transduction pathways of TKs in the detrusor muscle to provide potential pharmacological targets for the treatment of bladder dysfunctions related to enhanced TK production.
Tachykinins released in the bladder can act on: 1) NK1 receptors in blood vessels to induce plasma extravasation and vasodilation; 2) NK2 receptors to stimulate the bladder contractions; and 3) NK2 receptors on primary afferent terminals to increase excitability during bladder filling or during bladder inflammation. Substance P also acts on receptors on urothelial cells to release nitric oxide. Intrathecal administration of NK1 antagonists increased bladder capacity in normal conscious rats without changing voiding pressure, whereas NK2 antagonists were ineffective. Bladder hyperactivity in rats was also suppressed by intrathecal injection of NK1 antagonists. Bladder hyperactivity induced by capsaicin was reduced by an NK2 antagonist (MEN 11,420) that did not influence normal voiding. TAK-637, which is a highly specific antagonist for the NK1 receptor, is also reportedly effective to suppress bladder activity in guinea pigs. The key advantage of tachykinin antagonists is that there is essentially no decrease in detrusor contractility and no residual urine or retention risk. The drug works on the sensory nerves innervating the bladder and not on the bladder itself. Would it not be lovely to have one drug that can help not only OAB but also irritable symptoms of BPH and interstitial cystitis and yet causes no dry mouth or risk of urinary retention?
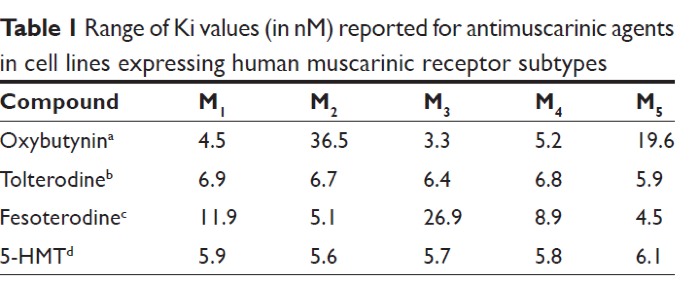
The muscarinic receptor has been the major peripheral pharmacological target in treating OAB. In the efferent pathway, acetylcholine, released from parasympathetic presynaptic nerve terminals binds to muscarinic receptors in the detrusor muscle to stimulate a detrusor contraction. Although there are 5 different muscarinic receptors located throughout the body (M1–M5), in the bladder, the M2 and M3 receptors predominate. The M2 receptor accounts for 80% of the muscarinic receptors in the detrusor M3 20%. The M3 receptor appears to have the primary role in normal detrusor contraction. M2 receptors appear to indirectly reverse sympathetically mediated smooth muscle relaxation. In certain diseased states, M2 receptors may also contribute to direct smooth muscle contraction. More recently muscarinic receptors have been identified in the urothelium and suburothelium.
Fesoterodine (Toviaz) Fesoterodine is a competitive muscarinic receptor antagonist indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency. This compound and also others antagonists act on all 5 muscarinic receptors (M1-M5).
Contractions of normal human bladders are induced primarily by ACH released from cholinergic nerve terminals in the bladder. It has been reported that nonadrenergic, noncholinergic bladder contractions are induced by increases of ATP levels in the human bladder under pathologic conditions such as denervation, bladder outlet obstruction, or idiopathic urge incontinence.
ATP acts on two families of purinergic receptors: an ion channel family (P2X) and a G protein-coupled receptor family (P2Y). P2X3 receptors have also been detected in the wall of the bladder in a suburothelial plexus of afferent nerves. In P2X3 knockout mice, afferent activity induced by bladder distention was significantly reduced.21
In patients with idiopathic detrusor instability, numbers of detrusor P2X2 receptors were significantly elevated, whereas those of other P2X receptor subtypes were significantly decreased.22 There was no detectable purinergic component of nerve-mediated detrusor muscle contractility in normal women without instability. However, there was a significant (approximately 80%) purinergic contractility component in bladder of women with detrusor instability.
In conclusion, the purinergic pathway may be a novel target for the pharmacological treatment of OAB. P2X-receptor antagonists could modulate efferent/afferent activities to treat OAB if receptor subtype-specific agents become available.
Animal studies and clinical trials have shown that beta-3 agonists relax the detrusor muscle, thereby improving bladder filling and compliance. There is a significant improvement of the incontinence and voiding episodes in 24 hours as well as a significant improvement of the quality of life.
The beta-3 agonist Mirabegron was approved for the treatment of overactive bladder in Japan in September 2011 and in Europe and the United States in June 2012. Other beta-3 agonists, such as Solabegron and TRK-380, are still under investigation.
Experimental studies have demonstrated that beta-3 adrenergic receptor (β3AR) agonists result in relaxation of the smooth detrusor muscle in humans mediated by the stimulation of the enzyme adenyl cyclase, which leads to the accumulation of cyclic adenosine monophosphate (AMPc). In addition, β3-AR activation seems to inhibit detrusor contraction also due to the release of urothelium-derived inhibitory factor (UDIF).
